Yomura's
3Q Verification Excellence
A comprehensive approach to ensuring
unparalleled medical device quality and safety.
What is 3Q ?
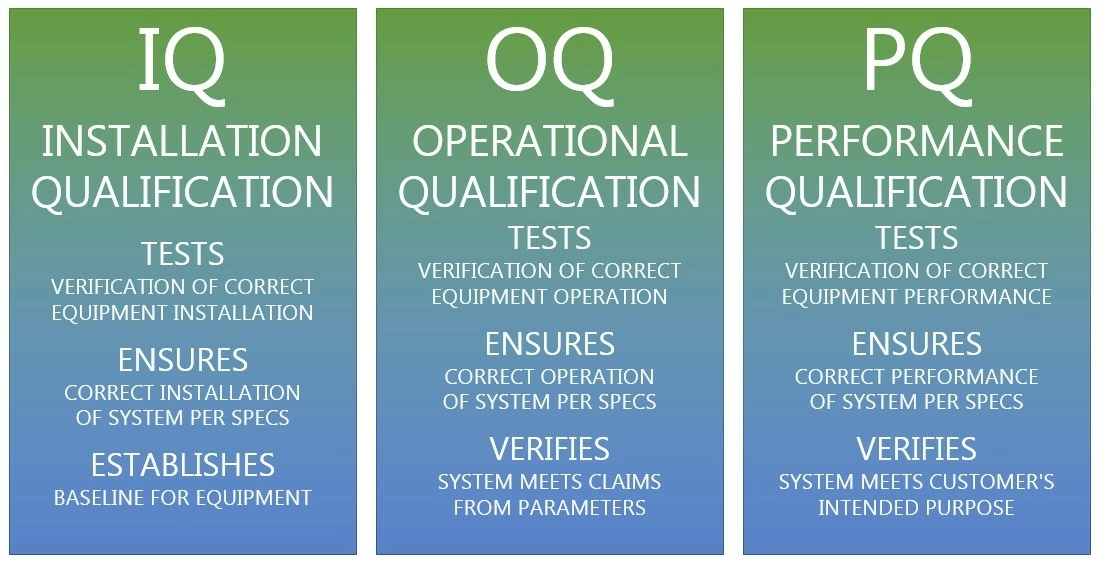
3Q stands for "Three Qualifications" which represent the three qualifying tests required for ensuring the reliability and performance of a piece of equipment or technology. These tests include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
IQ, Foundation of Assurance
Installation Qualification
Yomura's rigorous Installation Qualification forms the bedrock of our 3Q verification, encompassing meticulous inspections, precise calibrations, and exhaustive documentation to confirm every aspect of our medical devices are operationally ready.
Comprehensive Inspections
Yomura's rigorous Installation Qualification forms the bedrock of our 3Q verification, encompassing meticulous inspections, precise calibrations, and exhaustive documentation to confirm every aspect of our medical devices are operationally ready.
Calibration Certainty
Our highly-skilled technicians ensure that each component functions correctly through consistent calibration and cross-referencing with manufacturer standards to establish total system readiness.
OQ, Performance Safeguards
Parameter Assessment
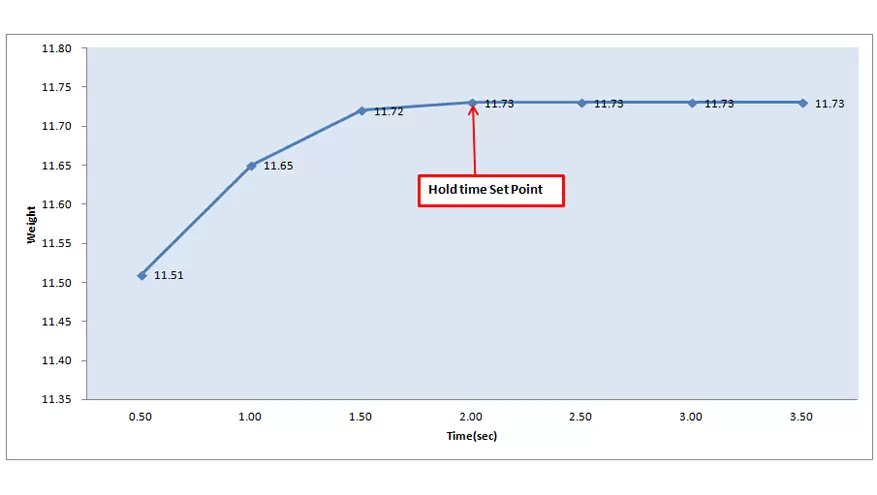
We subject our devices to extensive testing across all operational conditions, meticulously assessing variables like heat, pressure and timing, ensuring every performance criterion is met or exceeded.
Process Simulation
OQ simulation exercises test the devices' endurance, exposing them to the demands of actual usage scenarios to validate real-world reliability and efficacy.
Acceptance Framework
By establishing a detailed framework of predefined acceptance criteria, we leave no room for ambiguity in our pursuit of quality—each operational facet is verified against stringent thresholds.
PQ, Confirming Consistency
Production Runs
Yomura's PQ stage involves extensive production cycles, underpinning our steadfast commitment to manufacturing devices that are not just compliant but consistently excel in quality benchmarks.
Data-Driven Analysis
We collect, scrutinize, and analyze large volumes of production data, enabling precise and proactive quality control that is deeply informed by empirical evidence.
Regulatory Rigor
Aligning with global standards, our PQ process confirms that every device meets the most exacting quality specifications and regulatory imperatives, instilling deep trust in our manufacturing excellence.
Advanced QC Equipment Investment

Metrology Mastery
We harness the precision of advanced metrology tools to measure and ensure the utmost accuracy within every component of our medical devices.

Vision Inspection Innovations
State-of-the-art visual inspection systems provide an additional layer of scrutiny, capturing minute details unseen by the naked eye to reinforce our impeccable quality standards.

Testing Equipment Excellence
Meticulously maintained and calibrated testing apparatus ensures that each device undergoes a thorough evaluation to withstand the highest demands of performance.
Scientific Molding for Optimal Outcomes
Process Monitoring
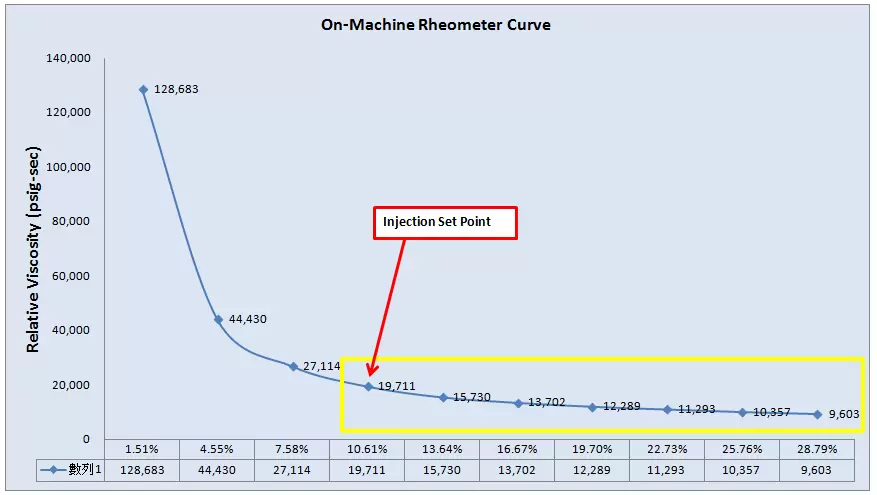
Intensively meticulous monitoring of the manufacturing environment and variables ensures predictability and consistency in production.
Data Analysis
Data is the nucleus of decision-making. Our rigorous analysis enables us to understand and eliminate process variations that could compromise quality.
Statistical Control
Employing statistical tools, we forecast, adapt, and continuously refine our processes for unerring precision and superior product outcomes.
Ongoing Commitment to Excellence
1
Continuous Improvements
Our relentless pursuit of excellence means never resting on laurels; instead we monitor, analyze, and improve our processes continuously.
365
Annual Audits
We subject our entire operation to rigorous audits yearly, ensuring enduring adherence to the highest quality and safety standards.
100%
Total Satisfaction
Our ultimate goal is the complete satisfaction of our clients and the health and safety of end-users, achieved through unwavering diligence in quality.